The solution, the science and support all in one place
Signant Biotech

Get from research to real world
Biotech and emerging biopharmaceutical organizations drive innovations in healthcare through novel therapeutic discovery and development. But with many time-sensitive goals and priorities to manage, these clinical development programs need comprehensive solutions, on-demand expertise, and efficient resources to achieve research milestones.
That’s where Signant can help.

Experience matters
We understand the importance of every trial, and every milestone, to emerging biopharma companies. As the leading evidence generation company with 25+ years’ experience, we’ve delivered from simple local trials to multinational pivotal studies. We've been there and back, and we're here to help.
No Delays
Because every milestone matters, trust Signant to deliver high quality, on time, every time
No Disconnects
Trust Signant as your single partner for end-to-end eClinical technologies, scientific support, and mature, global operational delivery services that flow seamlessly
No Doubt
Rely on Signant to optimize your measurement strategy, deliver solutions tailored to your protocol, and deliver the highest quality clinical evidence
How we support clinical development for biotechs & emerging biopharma
On-demand expertise

Supplement your team with clinical, scientific, and operational experts available in your time zone, reducing burdens on your team and facilitating smooth collaborations
Collaborative design
Leverage our medical and clinical experts to assist in early protocol development and design, ensuring protocols are optimized to generate reliable data for any indication while simplifying participation
Comprehensive technologies
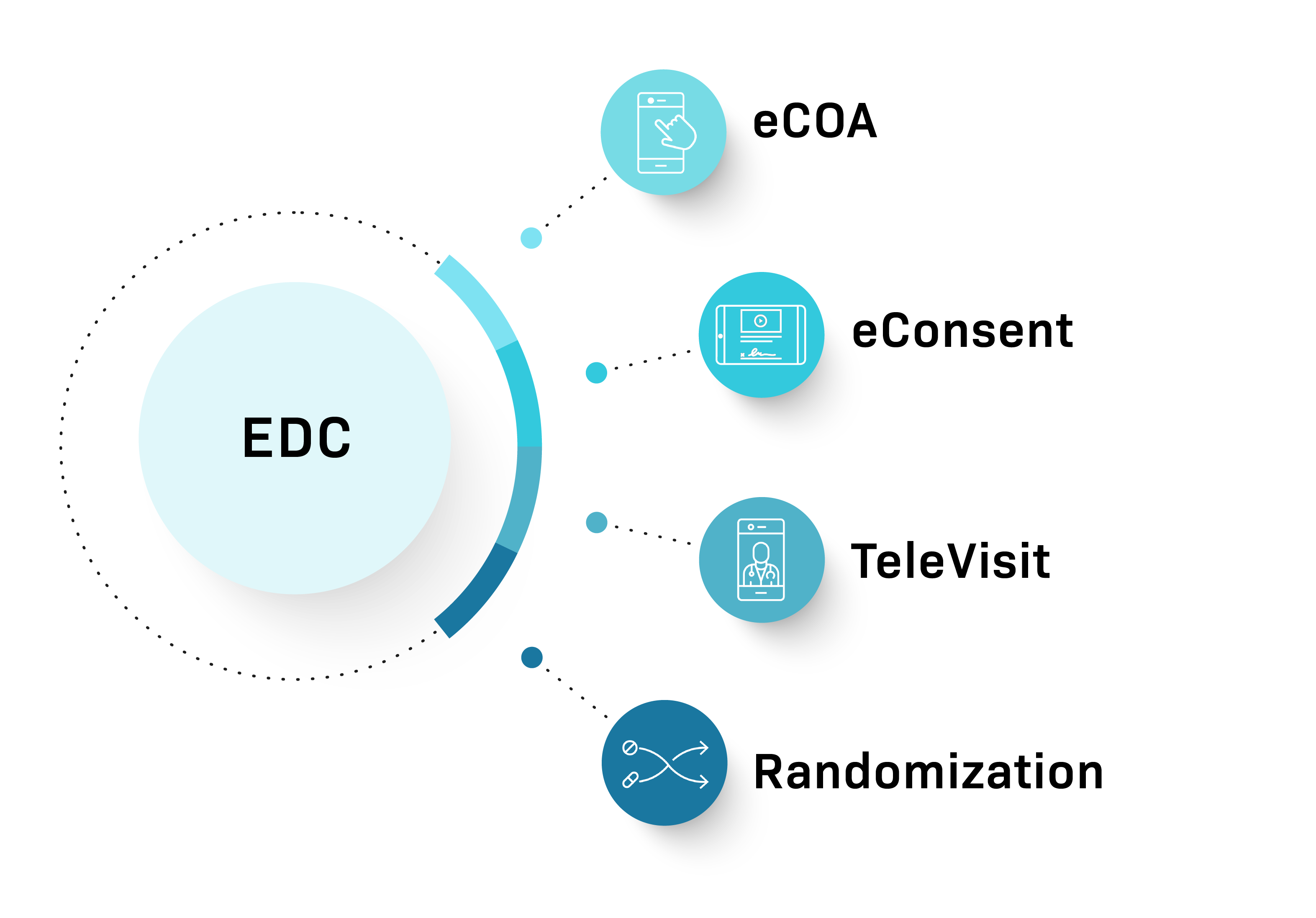
Our clinical trial experts tailor technology to your specific study needs—from our Unified Platform EDC with add-on modules for eCOA, eConsent, TeleVisits, and Randomization, to our specialized TrialMax, Rater Station, SmartSignals RTSM, and eConsent solutions for complex protocols.

Supplement your team with clinical, scientific, and operational experts available in your time zone, reducing burdens on your team and facilitating smooth collaborations
Leverage our medical and clinical experts to assist in early protocol development and design, ensuring protocols are optimized to generate reliable data for any indication while simplifying participation
Our clinical trial experts tailor technology to your specific study needs—from our Unified Platform EDC with add-on modules for eCOA, eConsent, TeleVisits, and Randomization, to our specialized TrialMax, Rater Station, SmartSignals RTSM, and eConsent solutions for complex protocols.
The solution, the science and the support all in one place
ONE PARTNER,
MANY SOLUTIONS
Work with one partner for end-to-end technologies, scientific support, and mature, global operational delivery services
ANY INDICATION
OR TRIAL DESIGN
Our nearly 25 years of experience means we are prepared and well-resourced to handle any study from simple, local trials to multinational pivotal studies
SPEED &
COST-EFFECTIVENESS
Engage us early to ensure you meet project and investment milestones on time and on budget
One integrated technology platform, tailored for emerging biopharma
Signant's Unified Platform starts with our comprehensive EDC solution at its core, with optional add-on modules that integrate seamlessly when needed. Our EDC foundation contains all the functionality you’d expect from a leading EDC solution.
Our fully embedded add-on modules include:
eCOA
Capture patient-reported outcomes directly with our intuitive, device-agnostic interface using BYOD or provisioned devices.
eConsent
Streamline the consent process with digital forms, remote review options, and signature capture.
TeleVisit
Simplify patient follow-up with secure, compliant video visits.
Randomization
Easily implement patient randomization within the EDC workflow.
A platform designed for biopharma
Streamline your clinical trials with Signant SmartSignals® Unified Platform. Discover how Unified Platform EDC and its add-on modules optimize data quality and trial operations without extending lead times or complicating user experience. Perfect for emerging biopharma, our integrated suite is tailored to meet the unique needs of sponsors, CROs, and sites in a competitive landscape. Watch the video to learn more.
Backed by Signant Health expertise
Science
Logistics
Translations
Our experienced team manages any new or adapted translations required, working with experienced, preferred translation vendors
Site and Patient Helpdesk
Patients can call our 24/7 multilingual helpdesk to gain help using our solutions
Scale Management
Our experienced scale management team leverages relationships with instrument owners and authors to ensure current and approved versions are implemented
Scale
Operational scale and global reach of Signant Health
Want to learn more?
Learn how Signant Biotech accelerates & streamlines clinical development