Signant Health's Unified Platform delivers the perfect balance of power and simplicity - a comprehensive EDC solution that accelerates study timelines without sacrificing quality or compliance, with modular add-ons tailored to your study needs.
Unified eClinical Platform

Discover a simpler experience without compromise
Streamlined Data Capture
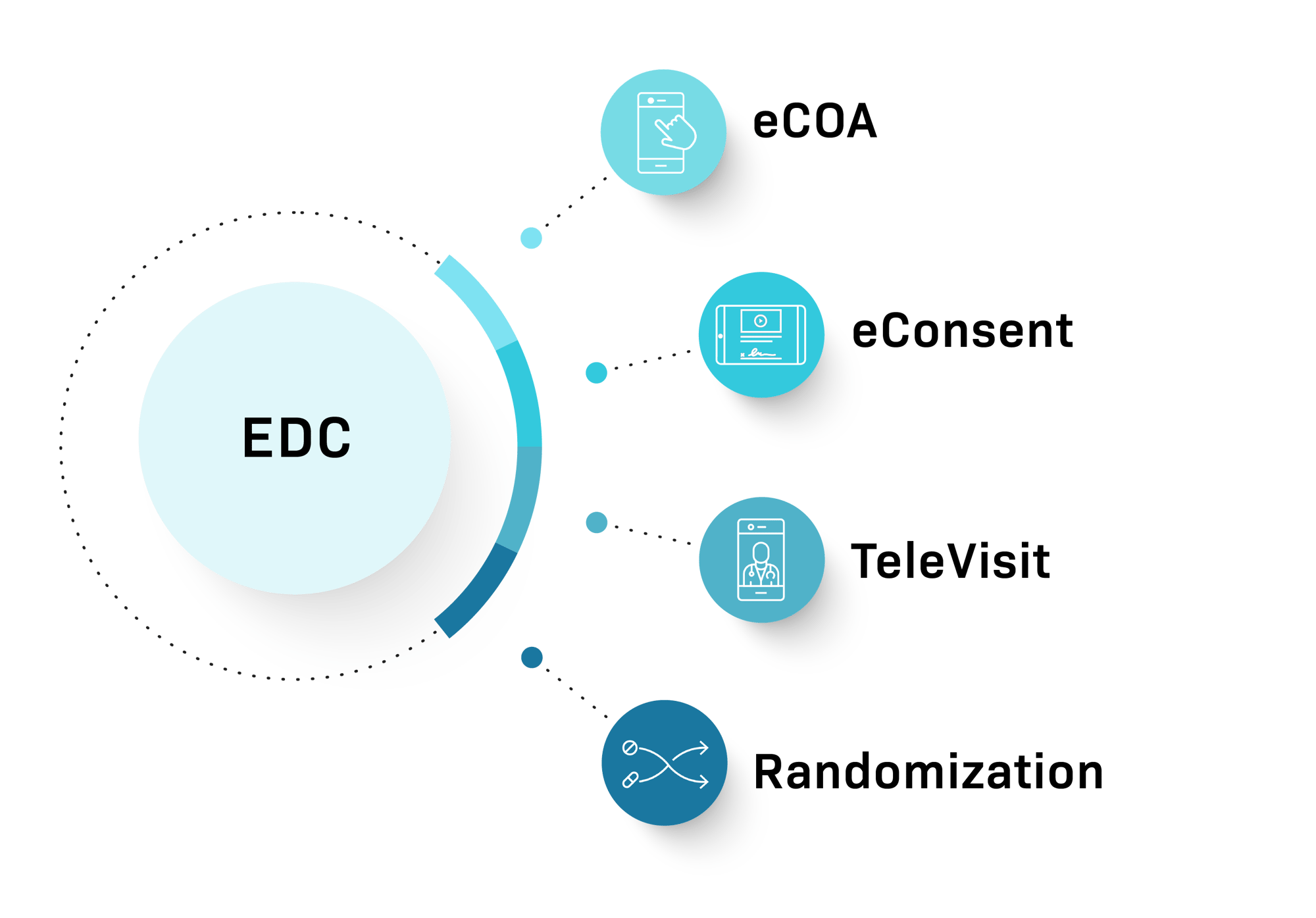
Signant SmartSignals® Unified Platform delivers a unified and fully integrated platform of key technologies to streamline data capture and trial management. With a robust EDC foundation, customers can choose to add eConsent, eCOA, Randomization and TeleVisits depending on their protocol needs.
Single Database
A single, configurable designer is used to build each EDC study and add-on modules (eConsent, eCOA, Randomization, and TeleVisits). Built on a single database, there is no need to provide back-end integrations between platform modules. Further, users benefit from single sign on, and workflows that enable invisible movement between modules.
Trusted Support
The platform has successfully supported more than 3,000 clinical trials, from simple to the most complex, throughout its 30+ year history, so you can be confident it will support your protocols and programs.
EDC that grows with you
Signant's Unified Platform starts with our comprehensive EDC solution at its core, with optional add-on modules that integrate seamlessly when needed. Our EDC foundation contains all the functionality you’d expect from a leading EDC solution.
Our fully embedded add-on modules include:
- eCOA. Capture patient-reported outcomes directly with our intuitive, device-agnostic interface using BYOD or provisioned devices.
- eConsent. Streamline the consent process with digital forms, remote review options, and signature capture.
- TeleVisit. Simplify patient follow-up with secure, compliant video visits.
- Randomization. Easily implement patient randomization within the EDC workflow.
Reasons sponsors & CROs love our Unified Platform
Sponsors and contract research organizations have been asking for a simpler experience without compromising data quality, user experience, or functionality. That’s where our SmartSignals® Unified Platform can help.
Simplified User Experience
- A single access point and a single set of user credentials enables access to our EDC and its add-on modules, eConsent, eCOA, TeleVisits and Randomization through a single URL and a single sign on
- Converged, guided workflow means users move invisibly between applications – simplifying the user experience
- Reduced site training requirements
- Reduced site burden means more time for mission-critical study activities and more time with patients
Rapid Setup
- A single configurable designer enables EDC implementation in just 4-6 weeks
- Highly-configurable, no-software-coding designer tools accelerate study builds
- A growing library of eCRFs with standard edit checks facilitates easy reuse
Self- or Full-Service Models
- A single, intuitive, no-software-coding designer enables Sponsors and CROs to easily design, configure, and put live their own studies; and apply seamless mid-study changes; all with minimal training
- Self- or full-service implementation models empower study teams to choose the best approach for their needs and goals
Expertise and Scale of Signant Health

- Science – Our experienced science and medicine, eCOA science, data management, and in-house biostatistics teams ensure optimal study design and best-practices are implemented
- Logistics – Where needed, our in-house logistics service ensures all sites and patients across the globe receive fully-configured and tested eCOA devices on time
- Translations and scale management – Signant experts work with eCOA scale authors and translation vendors to ensure right scales, with right versions, and reliable translations
- Site- and patient-facing helpdesk – Our 24/7, multilingual helpdesk ensures sites and patients have the support they need, when they need it
- A single access point and a single set of user credentials enables access to our EDC and its add-on modules, eConsent, eCOA, TeleVisits and Randomization through a single URL and a single sign on
- Converged, guided workflow means users move invisibly between applications – simplifying the user experience
- Reduced site training requirements
- Reduced site burden means more time for mission-critical study activities and more time with patients
- A single configurable designer enables EDC implementation in just 4-6 weeks
- Highly-configurable, no-software-coding designer tools accelerate study builds
- A growing library of eCRFs with standard edit checks facilitates easy reuse
- A single, intuitive, no-software-coding designer enables Sponsors and CROs to easily design, configure, and put live their own studies; and apply seamless mid-study changes; all with minimal training
- Self- or full-service implementation models empower study teams to choose the best approach for their needs and goals

- Science – Our experienced science and medicine, eCOA science, data management, and in-house biostatistics teams ensure optimal study design and best-practices are implemented
- Logistics – Where needed, our in-house logistics service ensures all sites and patients across the globe receive fully-configured and tested eCOA devices on time
- Translations and scale management – Signant experts work with eCOA scale authors and translation vendors to ensure right scales, with right versions, and reliable translations
- Site- and patient-facing helpdesk – Our 24/7, multilingual helpdesk ensures sites and patients have the support they need, when they need it
See for yourself
Unified Platform EDC and its add-on modules are all accessed through a single access point and using a single logon, and they are implicitly integrated, with no back-end data integrations needed.
Unified brings more benefits to sites through an uninterrupted workflow – users can move frictionlessly between all components (e.g., EDC, Randomization, eCOA, and eConsent) to complete their workflow.
Watch the demo to see how our Unified Platform simplifies workflow for site users.
Explore EDC and Add-On Modules
EDC
Our EDC module is a full-featured data capture solution with the ability to fully support modern trial designs including the option of eSource data capture, remote SDV, and risk-based monitoring support.
eCOA
Combining our EDC with our eCOA add-on module provides a consumer-grade patient experience and supports ePRO, eObsRO, and eClinRO capture both at site and at home.. Completion reminders and reporting drive on-time data collection, and biometric authentication simplifies the patient experience.

Randomization
Adding our Randomization module provides robust and reliable randomization and fully embedded within the EDC workflow. Combined with rapid and transparent emergency unblinding, this simplifies site staff activities and maintains trial integrity.

eConsent
Including our eConsent add-on module enables seamless transition from consenting to screening within site workflow. Simplifying the process of consent and reconsent, our module can be used for fully remote or on-site consenting.
Want to learn more?
Get in touch with us for a platform tour and to discuss your data capture and trial management strategy
