Solutions and services to generate quick, reliable results for decision making
Optimize Your
Phase I Clinical Trials

A technology approach tailored to the unique challenges of Phase I research
Phase I studies are typically small studies, rapidly set up, rapidly recruited, and rapidly completed. For this, researchers need data capture solutions that are agile, flexible, provide rapid access to data for decision making, and drive efficiency in implementation through re-use.
Introducing Signant SmartSignals® Unified Platform and other technologies to streamline early phase research.
Driving Efficiency
With Unified Platform
Signant SmartSignals Unified Platform is a comprehensive data capture solution that accelerates study timelines without sacrificing quality or compliance. With add-on modules, we can tailor our solution to your study needs.
Quick setup
Implement your study rapidly - in 4-6 weeks, or less
No downtime
No down time for system changes due to protocol amendments, so sites and CRAs can continue to work unaffected by mid-study updates
Remote monitoring
CRAs can save travel time by performing remote SDV
Direct data capture
eSource data capture with responsive design for mobile entry during patient consultations, site visits, or home visits, including comprehensive data genealogy tracking for appropriate SDV and data management activities
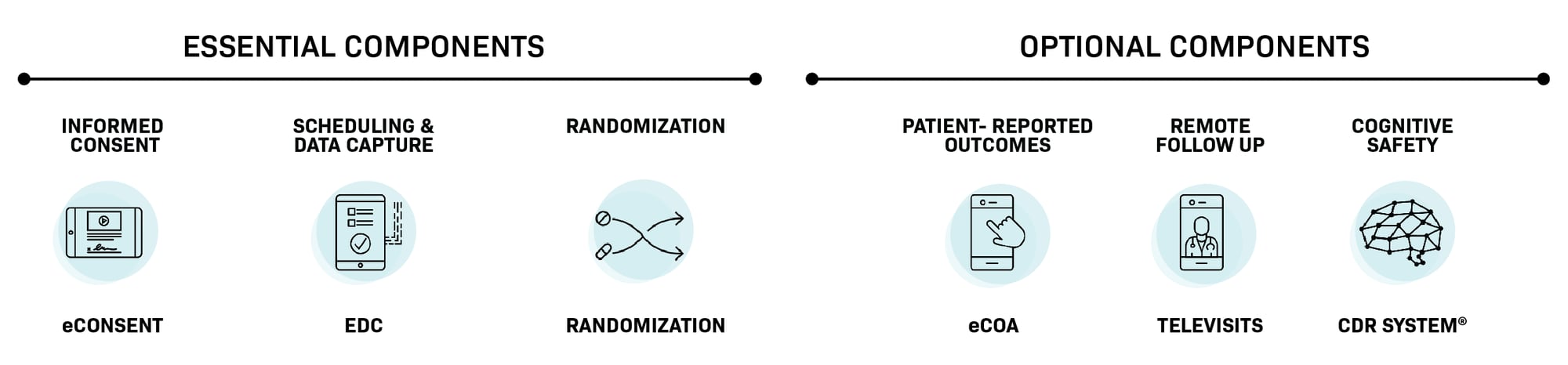
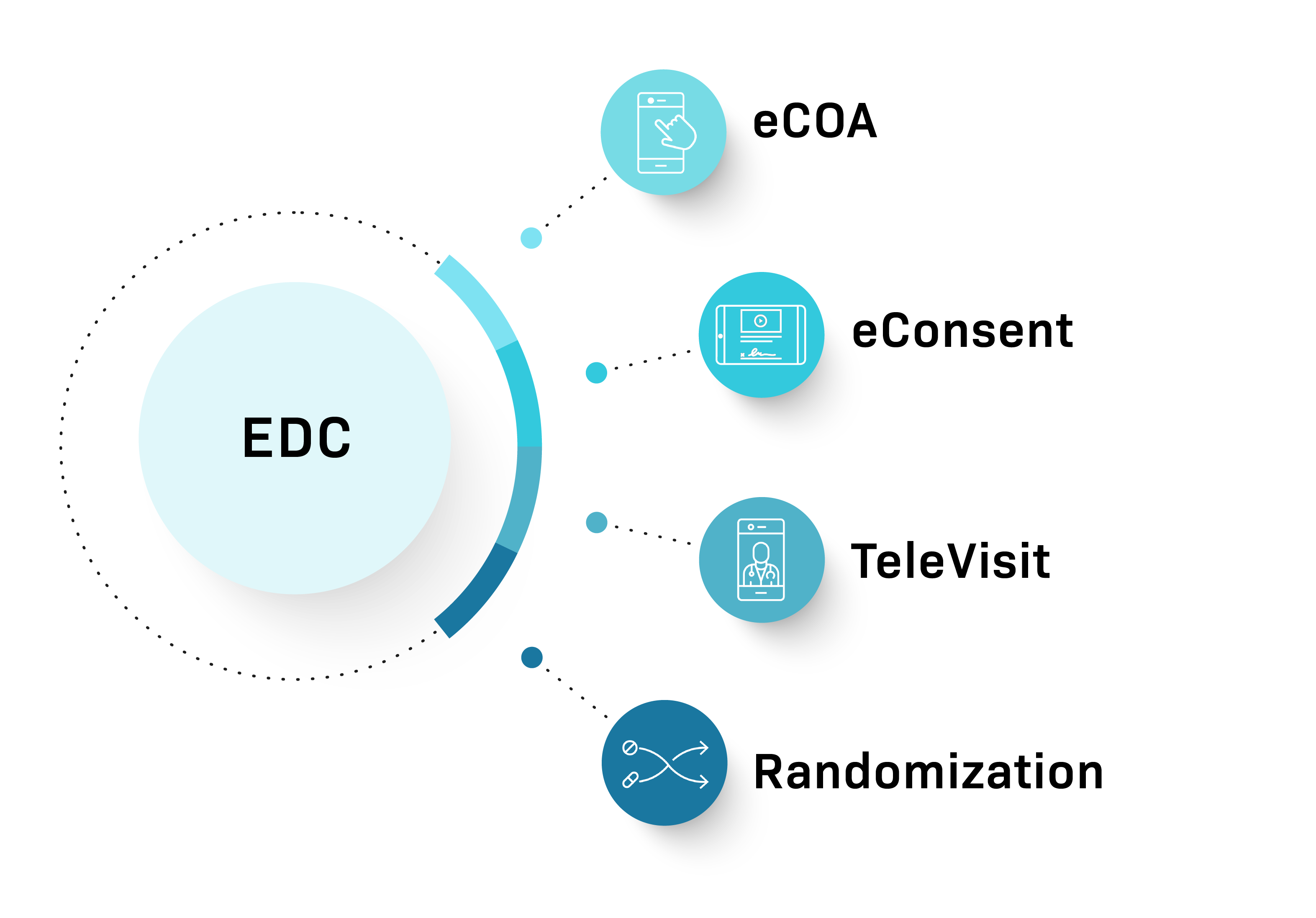
Comprehensive solution
Comprehensive modular platform offering eConsent, eCOA, TeleVisits, and Randomization with streamlined management through a single contract and dedicated project manager
Reliable system
From direct integrations with card networks and banks to checkout flows in the browser, we operate on and optimize at every level of the financial stack
Why choose Signant SmartSignals?
Experience
We’ve supported more than 700 phase I trials across many disease indications and therapeutic areas.
Unmatched Scientific Expertise
More than 50 full-time clinical and digital health sciences experts are available to support study teams from protocol design through study closeout.
Operational Agility
Our seasoned project teams help sponsors and CROs get high-quality results quickly with an eye toward later-stage success. Empowering self-service capabilities facilitates Phase I units to seamlessly integrate our solution into their operational workflows, fostering operational agility.
Integrated Solutions
Fully integrated Participant Tracker, eConsent, EDC, and RTSM solutions can be deployed quickly. Optionally, pair them with eCOA and TeleVisits for a digitally optimized Phase I trial.
FAQ
Why do Phase I clinical trials need eClinical technology?
Phase I clinical trials can gain the benefits of digitalization with electronic data capture (EDC), randomization, and electronic informed consent (eConsent) solutions driving rapid study start up, execution and data management. Using an integrated platform such as Signant SmartSignals® Unified Platform supports digitalization without slowing down trial operations.
What is Signant SmartSignals Unified Platform?
How does Signant SmartSignals® streamline Phase I trials?
Signant SmartSignals® streamlines Phase I trials by digitizing data collection, management, and analysis, ensuring real-time access to data and reducing errors. Alongside enhancing participant engagement through features like eConsent and remote monitoring, it also boosts operational efficiencies, expediting trial processes while maintaining high data quality and regulatory compliance.
How long does it take to set up Signant SmartSignals® for Phase I trials?
Want to learn more?
Get in touch with us and see how Signant can support your next Phase I study